The Women's Health Clinical Trial Experts
From study design to final reporting, Atlantia's end-to-end CRO for women's health trials delivers everything you need to take your product to market
40,000 Participant Database
Enter new markets or grow existing ones with our end-to-end Women's Health trials
Atlantia Clinical Trials drives progress in women's health clinical research, delivering results since 2011. We operate our own clinics in Ireland and the USA and have completed over 200 successful trials. As a trusted full-service CRO for women's health clinical trials, we partner with global sponsors looking to enter or grow within the European and American markets.

What's included in our Women's Health clinical research?
Our expertise in women's health clinical trials covers conditions like hormonal balance, osteoporosis, reproductive health and more. Addressing the unique physiological needs of women, Atlantia's in-house clinics and experienced teams execute trials with precision. We generate impactful insights and work with you to prove the efficacy of your new and existing products.
Clinical Services
Study Design & Compliance
Patient Recruitment
Study Conduct
Analysis & Reporting
Trial Models
Remote Trials
Hybrid Trials
Onsite Trials
PK / Bioavailability Studies
Research Sites
Atlantia Chicago Clinic
Atlantia Cork Clinic
50+ Partner Sites Across EU & USA
International Medical Network
Tailored Women's Health Research
We design customized women's health clinical trials using advanced technology, comprehensive biomarkers, and precise measurements. The end result is meaningful data that addresses the unique health challenges women face.
Some of the technology we use within women's health studies include:
-
Bioelectrical Impedance Analysis
-
Blood Pressure Monitors
-
CT
-
DEXA
-
Flow Medicated Dilation
-
MRI
-
Oximeter
-
Shotgun Sequencing, Metabolomics, Transcriptomics
-
Smartwatches & Wearables
Some of the biomarkers we test include:
-
Creatinine
-
Glucose
-
IgA Tryptophan (Stress Indicators)
-
Inflammatory Biomarkers
-
Iron Uptake
-
Microbiome Diversity
-
Omega-3 Fatty Acids
-
Oxidative Stress Measurements
-
Plasma Essential & Non-essential Amino Acids
-
Plasma & Salivary Cortisol
-
Sarcopenia & Amino Acid Uptake
-
Serum Triglyceride
-
Total protein, albumin, serum & urinary area
-
TSH
We use a range of measurements including:
-
Aerobic Capacity
-
Ambulatory Blood Pressure
-
Amsel's Criteria
-
Blood Pressure
-
Bone Density
-
Bowel Function
-
Endothelial Function
-
GI Transit
-
Kidney & Liver Function
-
Muscle Circumference, Max/Strength
-
Nugent Scoring
-
Resting Heart Rate
-
Sarcopenia & Amino Acid Uptake
-
STI Panel
-
Vaginal Infection - Symptom Duration, Episode Reduction, Symptom Severity
-
Weight
-
Anxiety & Sleep Quality
-
Biological Sample Indicators
-
Body Composition Measurements
-
Bone Health
-
Cardiovascular
Health -
Dietary Analysis
-
Gastrointestinal
Health -
Hormone Imbalance
-
Menopause
Symptoms -
Physical Activity & Performance
-
Pregnancy
-
Skin Health
-
Vaginal Infections
-
Well-being
The Atlantia Proof Playbook
From initial protocol to final data delivery, Atlantia's Proof Playbook guides you through every step of your clinical journey. Advance clinical trials in women's health and secure the evidence your product needs for commercial success.
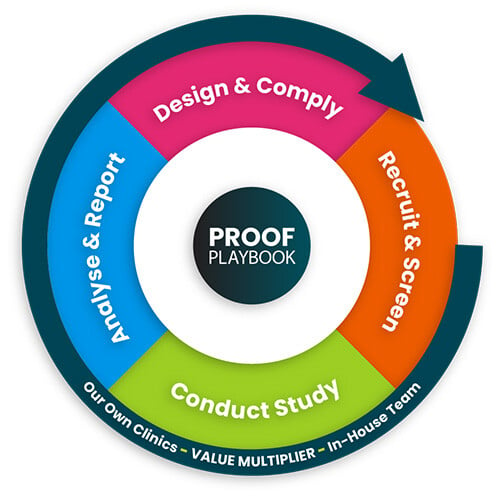
The Atlantia Proof Playbook
From initial protocol to final data delivery, Atlantia's Proof Playbook guides you through every step of your clinical journey. Advance clinical trials in women's health research and secure the evidence your product needs for commercial success.
Meet Our Dedicated Team

Aoife Lonergan
Senior Project Manager
.webp?width=600&height=600&name=Emma%20Harrington%20(2%20of%202).webp)
Emma Harrington
Clinical Research Coordinator

Stacey Boetto
Clinical Research Investigator

Dr. Nontokozo Yusuff
Medical Study Manager

Aoife Lonergan
Senior Project Manager
.webp?width=600&height=600&name=Emma%20Harrington%20(2%20of%202).webp)
Emma Harrington
Clinical Research Coordinator

Stacey Boetto
Clinical Research Investigator

Dr. Nontokozo Yusuff
Medical Study Manager
Advance your Women's Health clinical research in the EU and USA
Atlantia expanded to Chicago in 2019, growing our capacity to conduct multicenter women's health clinical trials. With a transparent approach and a commitment to data quality and timelines, Atlantia is recognized as a trusted partner in women's health clinical research. Collaborating with nutraceutical, medical device, cosmetics, and pharmaceutical sponsors of all sizes, we offer the infrastructure and expertise required for commercial success in the American and European markets.
Downloadable Resources



Ready to Lead the Market with Science?
Kickstart your women's health clinical program today.
Get in touch with our team to discuss your project.

