End-to-End CRO for Medical Devices
A dedicated specialist CRO team, with owned, operated, and integrated sites, delivering a faster path to market for clients.
Atlantia Clinical Trials is a global CRO with dedicated clinics in Cork, Ireland, and Chicago, USA. We run trials on-site, giving sponsors greater control over timelines, recruitment, and data quality, eliminating the need and cost of outsourcing. Alongside our expert in-house teams, we also have a trusted medical network we work closely with and can collaborate with a trusted network of medical specialists and research sites across Europe and North America if needed.
We support a broad range of medical device clinical investigations across diagnostic, therapeutic, and digital health technologies. Whether you're preparing a submission for the FDA, EU MDR, conducting post-market studies in the EU, or first in human studies, our team brings the regulatory and operational experience needed to support device trials across regions.
Why Choose Atlantia as Your Medical Device CRO?
Our model is built to address the everyday worries of device developers: speed, transparency, and reliable data.
- Regulatory experience – We support a range of submissions including 510(k), CE Marking, IDE, DeNovo, PMA and substantial equivalence studies.
- Global operations with local insight – Our teams in the U.S. and EU understand region-specific requirements under FDA, EUMDR, and IVDR.
- Clinics purpose-built for device studies – Our Cork and Chicago sites are equipped for a wide range of interventional and observational protocols, in addition to usability tests / human factor studies.
- Consistent senior oversight – Our leadership team remains actively involved throughout each trial.
- Flexible for small to mid-sized sponsors – We offer low investigator fees, no hospital overheads, reduced protocol deviations, and more patients per site, allowing you to control timing and budget, and a faster study start up time.
- Predictable recruitment – Our in-house recruitment team uses our own patient databases and community outreach, allowing us to hit enrollment targets on time.
- Faster study reporting – Our integrated clinical and data teams allow for efficient database lock and report generation.
Our Medical Device CRO Expertise
Atlantia combines scientific depth with operational precision to provide the best medical device CRO services available to fit a variety of budgets. Our expertise in medical device CRO solutions spans every stage from concept protocols to real-world evidence.
- Full-service clinical development – feasibility, pivotal, post-market studies, procedure follow-up, and real-world evidence
- Regulatory strategy & submissions – 510(k), IDE, PMA, De Novo, CE Marking; MDR/IVDR readiness
- Medical writing & technical documentation – CER, PER, PMCF, PSP, IFU, Human Factor, Clinical Evaluation Plans
- Data management & biostatistics – tailored for device-specific endpoints
- Site management & monitoring – fast, low-cost start-up at our clinics; access to patients.
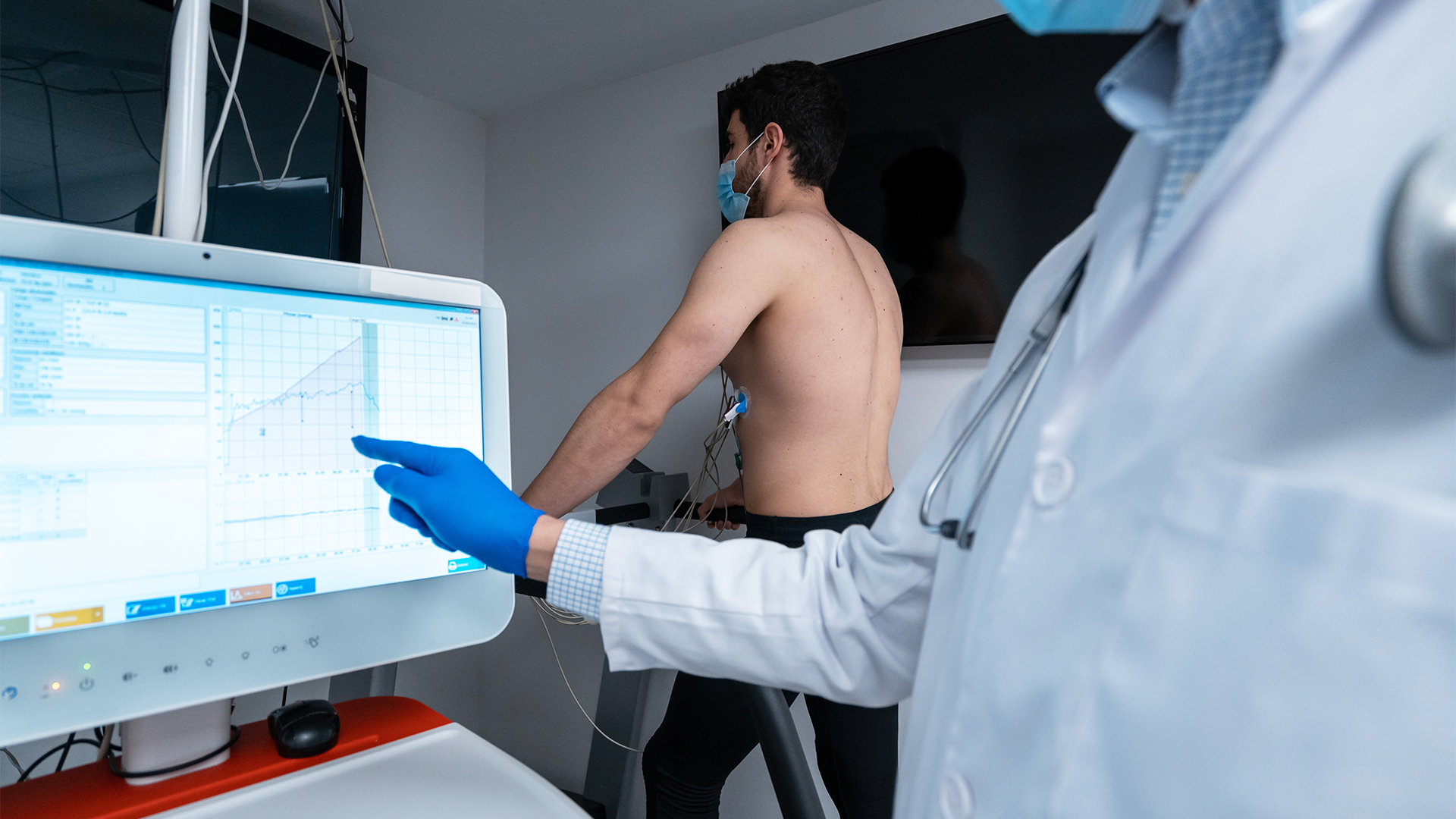
Who We Support
- Medical Device Manufacturer's - Class I, II, & III
- Diagnostic & Digital Health Compnaies
- Start-ups & emerging MedTech Teams
- International Firms entering the US & EU Markets
- Investigator-Initiated Trials (IITs)
- Cardiovascular
- Orthopedic
- Pain & Neuromodulation
- Digital Therapeutics
- Neurodegenrative
- Dermatology
- First-In-Human
- Sensors
- RPM (Remote Patient Monitoring)
- IVD (In Vitro Devices)
- ISO-Compliant Processes (ISO 14155:2020)
- ISO 9001 QMS
- ICH-GCP
- GDPR & HIPAA Data Management

Let's Discuss Your Device
FAQ
What types of medical devices do you support in clinical trials?
We handle implants, wearable sensors, software, diagnostics, and combination products.
Do you support both pre-market and post-market clinical studies?
Yes - feasibility, pivotal, surveillance, registry, and RWE trials are all supported.
Do you provide support for regulatory submissions like 510(k) or CE Marking?
Yes, including authoring and submission for 510(k), IDE, PMA, CE dossiers.
What regulatory pathways do you support?
510(k), IDE, De Novo, PMA, & CE (MDR/IVDR)
Can you manage global studies?
Yes, we can recruit patients from our European and US sites, but can also work in tandem with our partner hospital sites to deliver your study.
What makes your CRO different from others in the medical device space?
We own our sites, provide fast contracts, deliver fixed-cost quotes, and our expert staff are here to update you throughout the entire process from start to finish.
How do we get started with a project or request a proposal?
Click "Request a Proposal", email us, or schedule a call with our sales team.
