The Skin Health Clinical Trial Experts
From study design to final reporting, Atlantia's end-to-end CRO services include everything you need to get market-ready skin health clinical research results.
40,000 Participant Database
Enter new markets or grow existing ones with our all-in-one Skin Health trials
With international clinic space, and over 200 trials complete, Atlantia Clinical Trials is a trusted skin care and dermatology CRO. We provide end-to-end clinical research services to global sponsors aiming to break into the European and American markets or grow their existing share of these markets by advancing innovations in skin health clinical trials.

What's included in our Skin Health CRO services?
Our skin health research addresses a variety of conditions, including eczema, acne, and psoriasis, as well as cosmetic advancements. Atlantia’s in-house clinics and expert teams deliver reliable trial management, precise data collection, and participant safety. These capabilities enable sponsors to meet regulatory standards in both the U.S. and Europe with confidence.
Clinical Services
Study Design & Compliance
Patient Recruitment
Study Conduct
Analysis & Reporting
Trial Models
Remote Trials
Hybrid Trials
Onsite Trials
PK / Bioavailability Studies
Research Sites
Atlantia Chicago Clinic
Atlantia Cork Clinic
50+ Partner Sites Across EU & USA
Global Medical Network
Tailored Skin Health Clinical Trials
We design customized skin health clinical research trials using advanced technology, biomarkers, and measurements to test for a range of endpoints. Our results deliver reliable data that address specific dermatology challenges.
Some of the technology we use within stress & mood studies include:
-
Tewameter
-
Antera 3D
-
Profiling of Skin Microbiota
-
Lipidomics
-
Visiometers
-
Corneometer
-
Cutometer
-
Mexameter
Some of the biomarkers we test include:
-
Erythema & Melanin
-
Skin Surface pH
-
Trans-epidermal Water Loss (TEWL)
-
Epidermal Hydration
We use a range of measurements including:
-
Minimal Erythema Dose (MED)
-
STAR Classification System
-
Anti-wrinkle/Texture
- Firmness/Elasticity
- Dark Spots/Age Spots
- Skin Protection/Repair
- Skin pH
- Skin Brightening
- Anti-Sebum/Sebum Control
- Lip Dryness/Lip Balm
- Hydration/Moisture
- Eczema
- Skin Dryness & Presence of Skin Tears
- Measure of Purities
- Self-Reported Measure Quality of Life
- Dietary Analysis
- Skin Sampling
- Skin Microbiota Profile
- Skin Lipid Profile
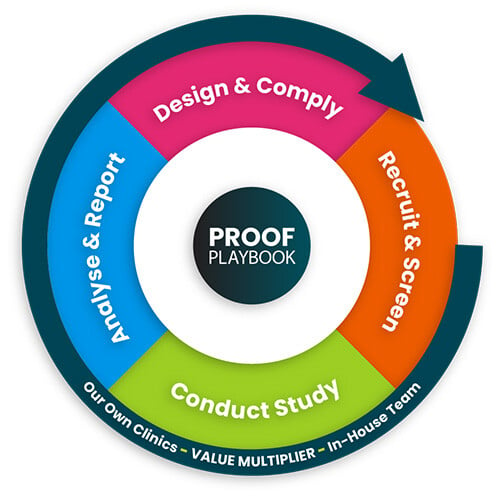
The Atlantia Proof Playbook
From initial protocol to final data delivery, Atlantia's Proof Playbook guides you through every step of your clinical journey. Advance clinical trials in skin health and dermatology and secure the evidence your product needs for market success.
Meet Our Dedicated Team

Aoife Lonergan
Senior Project Manager

Emily Goodbody
Senior Operations Manager

Kevin O'Regan
Senior Operations Manager

Lavita Vaz
Research Coordinator

Aoife Lonergan
Senior Project Manager

Emily Goodbody
Senior Operations Manager

Kevin O'Regan
Senior Operations Manager

Lavita Vaz
Research Coordinator
Expanding Skin Health Clinical Research into American and European Markets
Atlantia expanded to Chicago in 2019, growing our capacity to conduct for multicenter skin health clinical trials. With our transparent approach and commitment to data quality and timelines, Atlantia is recognized as a reliable dermatology and skin care partner for global nutraceuticals, medical devices, pharmaceuticals, and cosmetics sponsors. Whether evaluating a novel dermatological treatment or a new cosmeceutical product, Atlantia provides the infrastructure and expertise necessary to advance skin health research for sponsors looking to enter or grow within the European and American markets.
Downloadable Resources



Ready to Lead the Market with Science?
Kickstart your clinical program today.
Get in touch with our team to discuss your project.


